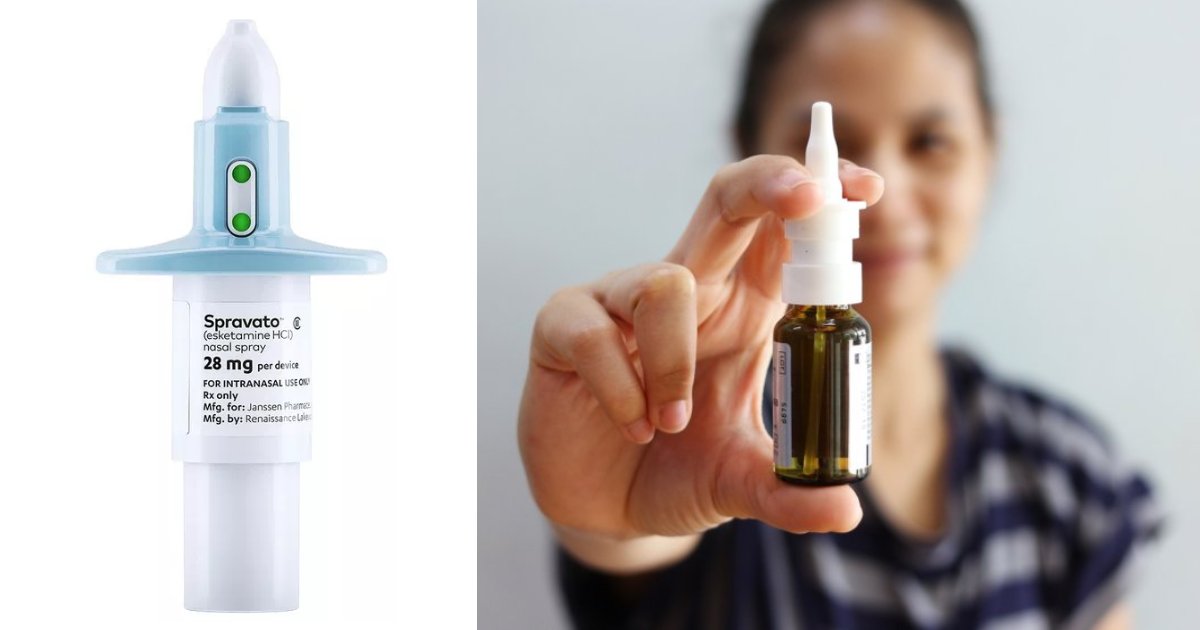
The United States Food and Drug Administration has approved a new medication related to the club drug Special K on Tuesday.
Spravato will be used for patients facing severe depression via a nasal spray mechanism.
The mind-altering drug contains a chemical like ketamine, which has been used for a long time as a pre-surgery powerful anesthetic for patients.
With the FDA’s approval of the nasal spray from Johnson & Johnson, patients who fail to find relief with at least two antidepressants will be able to get this fast-acting treatment.
According to the FDA, more than 7 million Americans suffering from treatment-resistant depression are at high risk of hospitalization, suicide, and other severe harms.
The new drug will be priced between $590 and $885, depending on the dosage and different rebates and insurance discounts.
Unlike most of the antidepressants, Spravato, chemically known as esketamine, works very fast and has almost immediate effects.

Dr John Mann, a psychiatrist and researcher at Columbia University, said its speed “is a huge thing because depressed patients are very disabled and suffer enormously.”
He also noted that doctors can quickly try other options if it doesn’t work.
Spravato’s effectiveness has been well-established, thanks to various case studies. For instance, it brought drastic changes in the life of 60-year-old Robin Prothro.
Prothro, who has been taking antidepressants for more than 20 years, says she has previously tried five medications but none of them relieved her depression.
But after she enrolled in a Spravato trial two years ago, her depression has almost ended and she has returned to a normal life, enjoying her long-abandoned hobbies like gardening.
She takes Spravato every two weeks while sitting in a comfortable chair at her psychiatrist’s office.
“You can feel it coming on, it’s a strong drug,” Prothro said of the drug. “I just let the drug work. I close my eyes and my mind is amazingly quiet.”
Despite its usefulness, J&J’s drug is subjected to wide-ranging restrictions due to its potential abuse, side effects, and other safety questions.
Only accredited specialists will be given the new drug and they will have to monitor the patients for at least two hours after subjecting them to the drug due to its disorienting and trippy effects.
Additionally, all patients who take Spravato will be recorded in a registry so that the drug’s long-term effectiveness and safety can be monitored.
J&J’s medication is expected to receive a welcoming response from Wall Street and several analysts have predicted it to make over $600 million in annual sales by 2022.
Recommended Video – “Firefighter Leans Out Window Just In Time To Catch Suicide Jumper In Dramatic Rescue”