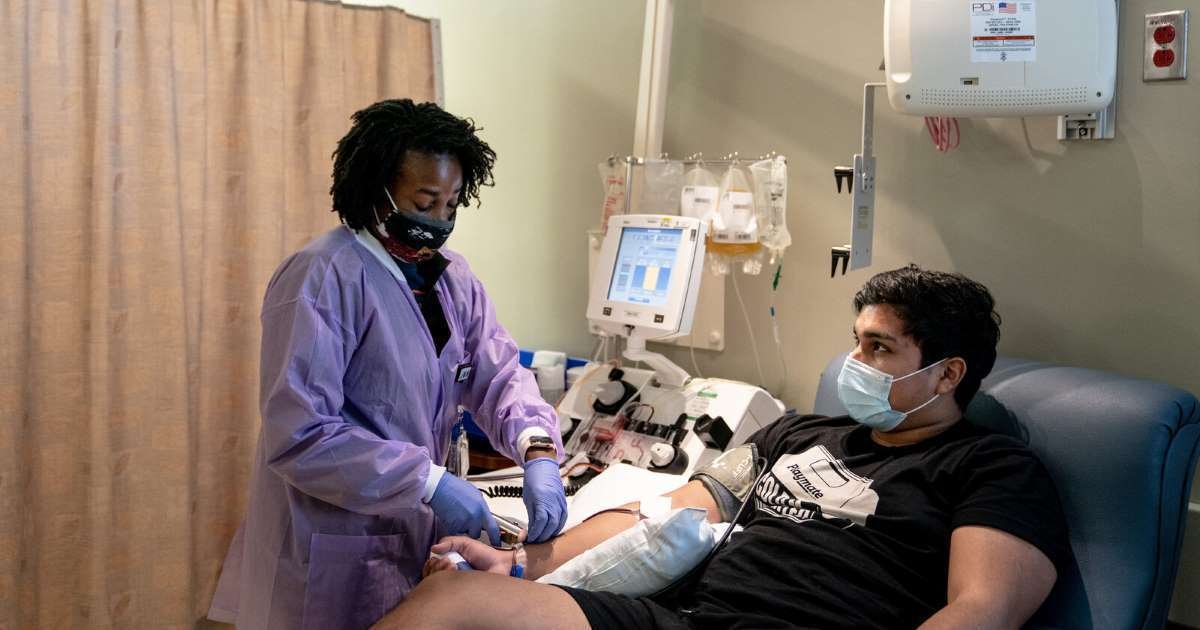
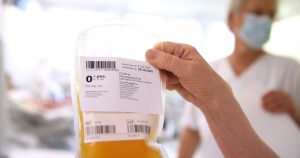
The US Food and Drug Administration on Sunday has announced that it has allowed in contingency usages for convalescent plasma to be utilized in treating Covid-19, saying the “known and potential benefits of the product outweigh the known and potential risks of the product.
” The FDA claims that as 70,000 patients had been treated with convalescent plasma, the bodily liquid extracted from the blood of people who have recovered from coronavirus infections, there is no reason not to use it in the most needed treatments.
“Today I am pleased to make a truly historic announcement in our battle against the China virus that will save countless lives,” President Trump said at a White House briefing, “Today’s action will dramatically increase access to this treatment.
” Trump had a lot to say about the subject since last week, mainly due to the fact that he believed health officials placed in the decision-making process has denied the okay sign for the plasma for political motivations. The FDA needs to issue a EUA grant on an experimental treatment to be dispersed in usage across the nation at a federal level.
On Sunday, it was made know that the FDA had called its staffs to review additional data to inform its EUA decision. The source involved vehemently denies any outside pressure, saying that the FDA is under no obligation to consult anyone outside the agency about its decision.
The plasma, despite questions arising from outside experts about its security, has already been applied in usage to almost 60,000 Covid-19 patients.
The nature and origin of convalescent plasma being in the blood supply of those who survived COVID-19, the novel treatment is in limited supply and must come from willing donors. There is not enough randomized clinical trial data on convalescent plasma to treat Covid-19.
At best, the plasma is yet to be given a clinical all-clear sign.
However, US Health and Human Services Secretary Alex Azar said studies had proven enough.
“The data we gathered suggests that patients who were treated early in their disease course, within three days of being diagnosed, with plasma containing high levels of antibodies, benefited the most from treatment. We saw about a 35% better survival in the patients who benefited most from the treatment,” Azar told the White House briefing.
“We dream in drug development of something like a 35% mortality reduction. This is a major advance in the treatment of patients. A major advance.”
If you liked this article, please LIKE SHARE AND COMMENT below! And don’t forget to check our other articles along the way!
Replaced!