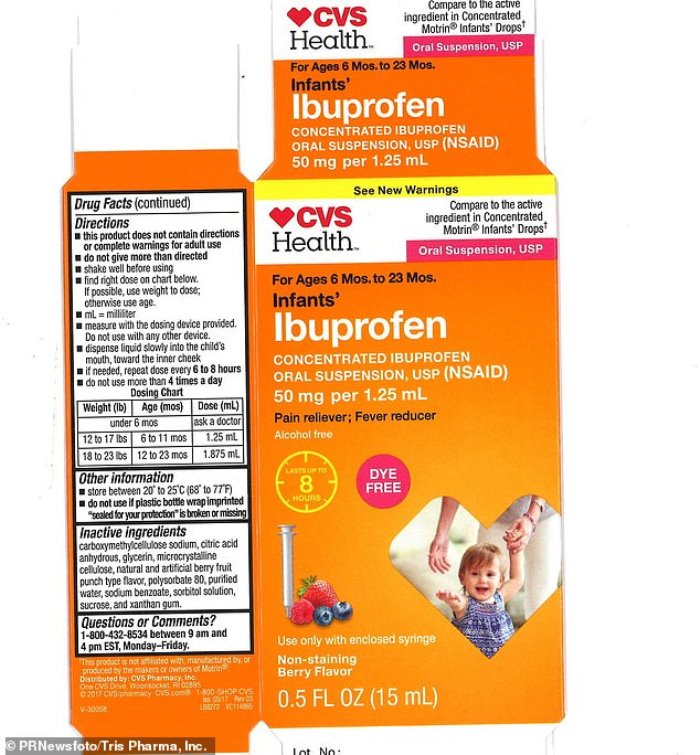
A pharmaceutical company has issued a voluntary recall of its infant liquid ibuprofen.
Tris Pharma, Inc from New Jersey says the recalled lots may have higher concentrations of ibuprofen that could cause permanent kidney or renal damage among babies.
In a statement, the company said that there is a ‘remote possibility’ that infants may experience side effects including stomach pain, vomiting, diarrhea, and nausea because of the increased potency.
Ibuprofen belongs to a type of drugs called nonsteroidal anti-inflammatory drugs (NSAIDs). These drugs temporarily lower the amount prostaglandins, a type of lipid that the body makes in cases of injuries.
The affected lots were sold at Family Dollar stores, CVS Pharmacy and several Walmart across the country.
The recalled ibuprofen were packaged in 0.5 oz. bottles and labeled ‘Infants’ Ibuprofen Concentrated Oral Suspension.’
According to the description, the drug is meant to be used as a fever reducer or pain reliever, containing 50mg per 1.25 mL.
Prostaglandins cause inflammation, pain, fever or swelling. But these lipids maintain body fluid pressure to let kidneys filter waste from the body. They also help protect the stomach lining from stomach acid.
Reduced amounts of prostaglandin production can cause decreased kidney function and may result in stomach damages, which leads to ulcers and bleeding.
CVS sells the drug under the label CVS Health. Walmart sells it under Equate and Family Dollar sells it under Family Wellness.
The recalled products have the lot numbers 00717024A from Family Dollar and 00717024A from CVS, both with August 2019 expiration dates.
From Walmart, the recalled products have lot numbers 00717009A, 00717015A and 00717024A. The expiration dates are February 2019, April 2019 and August 2019.
The National Drug Code numbers on the recalled packages are 55319-250-23 for Family Dollar, 59779-925-23 for CVS, and 49035-125-23 for Walmart.
Don’t forget to SHARE this info with your family and friends!